Cancer Research for Children
Project location: Italy
Project start date: January 2005 -
Project end date: This project covers various years
Project number: 2005-13
Beneficiary: AIRC - Associazione Italiana per la Ricerca sul Cancro
[2009-02] Interim Report
AIRC aimed to contribute to the molecular basis of MB and to design new therapeutic strategy
oriented to impair the stem-like proprieties of MB or/and the main aberrant pathways involved in the tumor progression and the chemo-resistance to cisplatin, observed in a sub-set of MB patients.
All together these comprehensive approaches underlie the importance of novel potential therapeutic strategies to enhance patient-personalized therapy in MB in the near future.
The main limitation of the current treatment of MB is the lack of drug specificity. The Prof. Zollo's team aimed to elucidate the signaling pathways involved in normal development of MB thus improving the clinical management of this neoplasia and suggesting new therapeutic tools to impair mortality and improve morbidity of MB patients. The team focused on the searching for specific impairment pathway drugs (WNT, NOTCH, SHH), making use of a new library of compounds both synthetic and natural. These compounds were screened for their ability to impair WNT and Notch pathways by using luciferase assay methods and to slow down tumorigenesis on MB cell lines. Three interesting drugs were identified and tested in vitro on three different MB cell lines to check the anti-tumoral activity. Norcantharidin (NCTD) in vivo efficacy was also tested in vivo in orthotopic intra-cerebellum xenograft animal models. These animals were injected with DAOY MB cell line previously stably transfected with luciferase gene and MB tumorigenesis were followed by in vivo imaging (BLI) technologies. Upon three treatments per week of the peritoneal administration for a total of 15 days, tumorigenesis was observed significantly decreased showing a neural-differentiation phenotype. These results have a translation therapeutic potential for two main reasons: 1) NCTD impairing WNT signaling induces a benign neuronal-differentiation and then 2) it could be administrated systemically by injections into peritoneal, since we show it is able to cross the blood brain barrier (BBB). A manuscript has been prepared and it will be soon submitted to a journal within this area of research. For the future planning, it is proposed to continue the screening
of natural and synthetic compounds looking for those impairing the Shh/GLI pathway in vitro and to test the anti-tumor activity in vivo by using xenografts animal models. The aim will be to identify new drugs which show a clear effect on the main aberrant pathways in MB and inhibition of the propagation of stem cells.
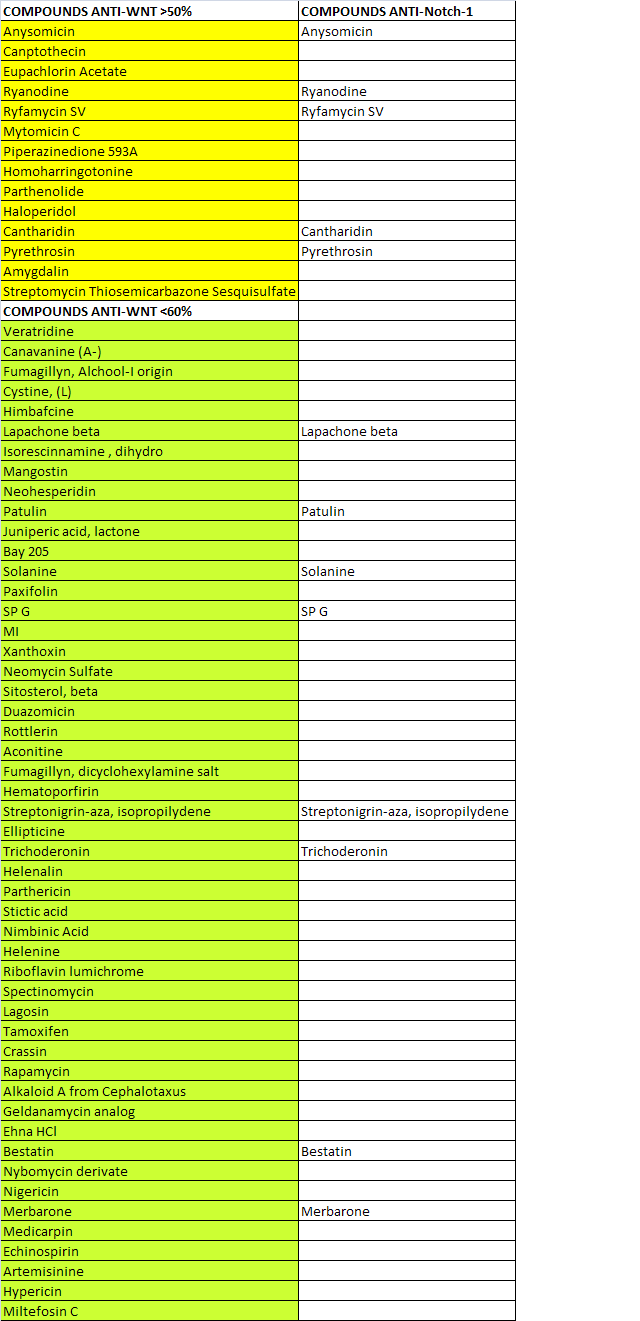
Table 1. 100 natural compounds were screened for their ability to impair WNT signaling by performing luciferase assay in Hek293 model system. N.64 screened natural compounds show anti-Wnt less than 60% with respect to control. N.14 of screened compounds show to impair Wnt signaling more than 50% . Some of these compounds have shown to affect additionally the Notch1 signaling.
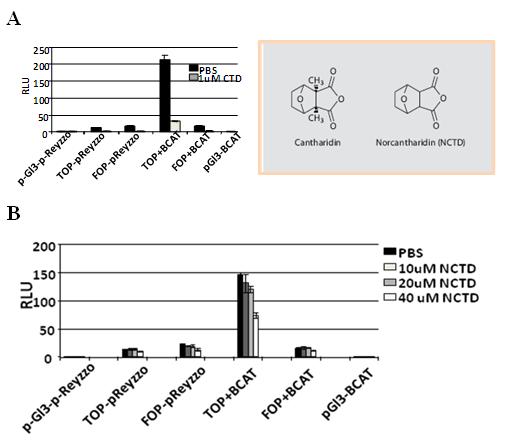
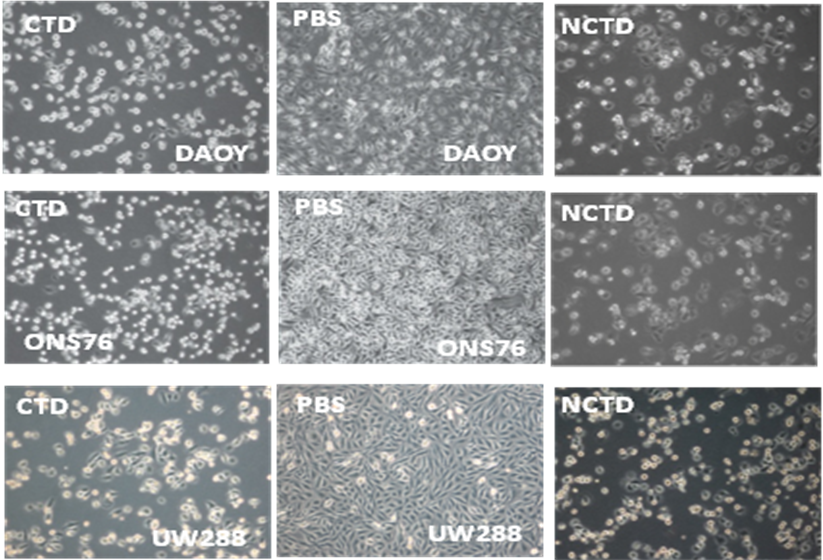
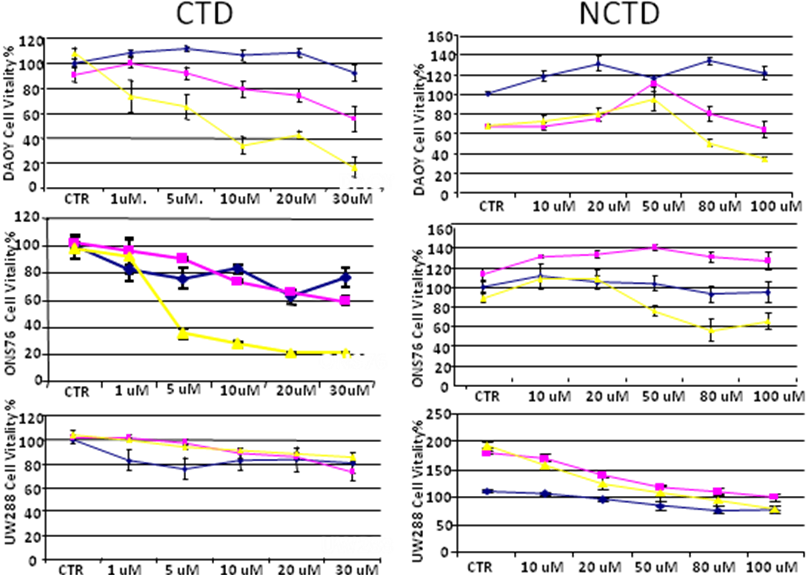
Figure 3. Three MB cell lines were treated with Cantharidin (CTD) and Norchantaridin (NCTD) at different concentration points and for 24h (blue line), 48h (pink line) and 72h (yellow line). Daoy cells rappresent a desmoplastic tumor, ONS76 and UW288 rappresent a classic type of tumors. CTD and NCTD were dissolved in PBS (CTR). The compounds induce anoikis, imparing cellular capabilities to attach the plate. Through MTS proliferation assay we determine the IC50 using the value at 72h.
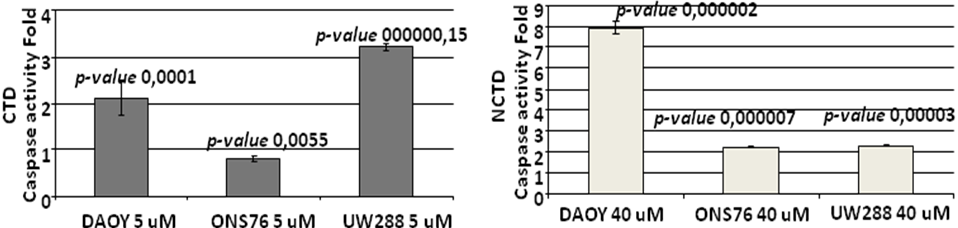
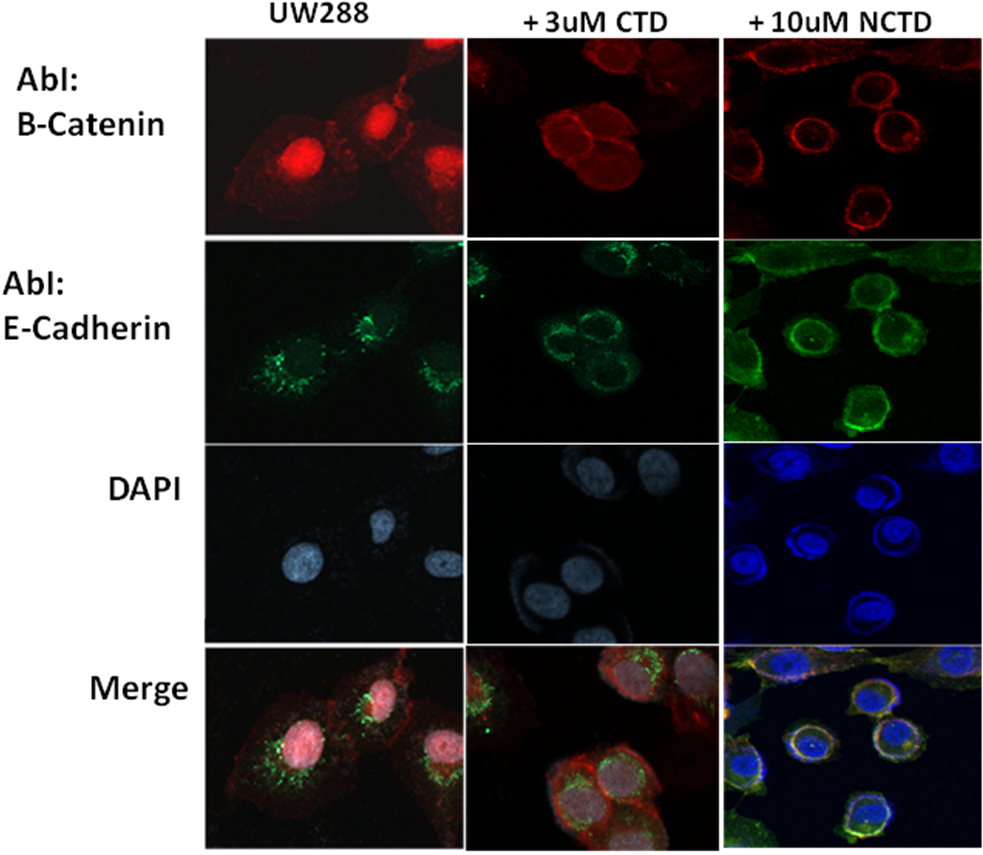
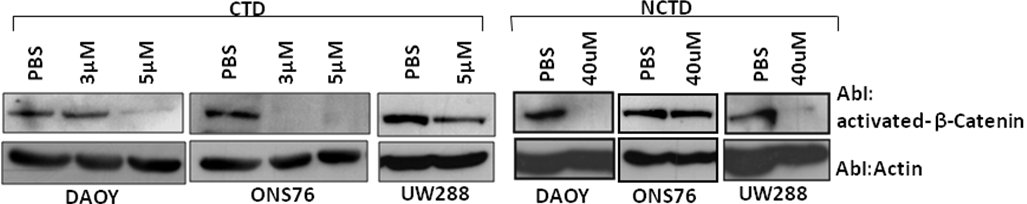
Figure 6. Upon treatment with CTD and NCTD at choosen concentrations, the Wnt signaling is impaired as shown by Western Blotting. Antibody anti activated β-catenin recognized amino acid residues (aa36-aa44) of human Catenin, specific for the active form, dephosphorylated on Ser37 or Thr41.
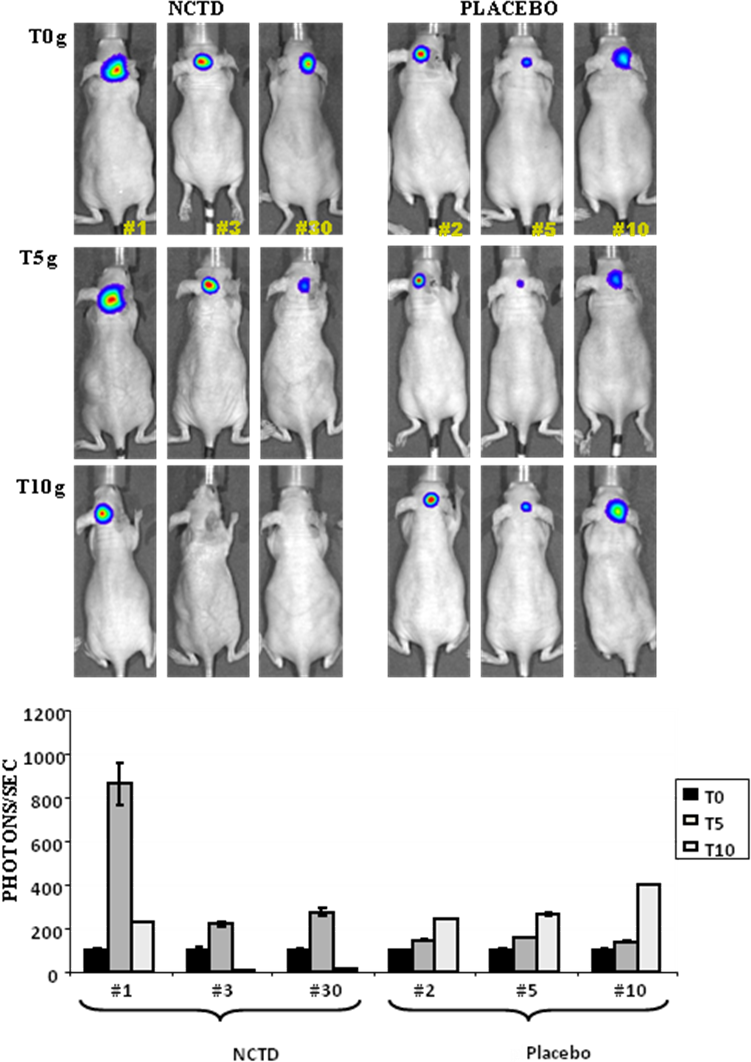
Figure 7. 3 x105 DAOY-LUC cells were injected intracerebellum in NOD/SCID mice at 6 weeks of age and analyzed for 20 days; the tumorigenesis was evaluated by in vivo Bioluminescence assays (BLI). The animals were divided in two groups; NCTD group (2mg/prokg/day; 7 days a week) presents BLI values higher than placebo animal group. The mice were then imaged at before (T0) and after treatment : 5 and 10 days analyses (T5, T10). The photons/sec were detected by IVIS-Spectrum, Calipers/Xeongen IVIS 3D Illumina instrument and for each animal the BLI values were normalized with respect to their own T0 value.




